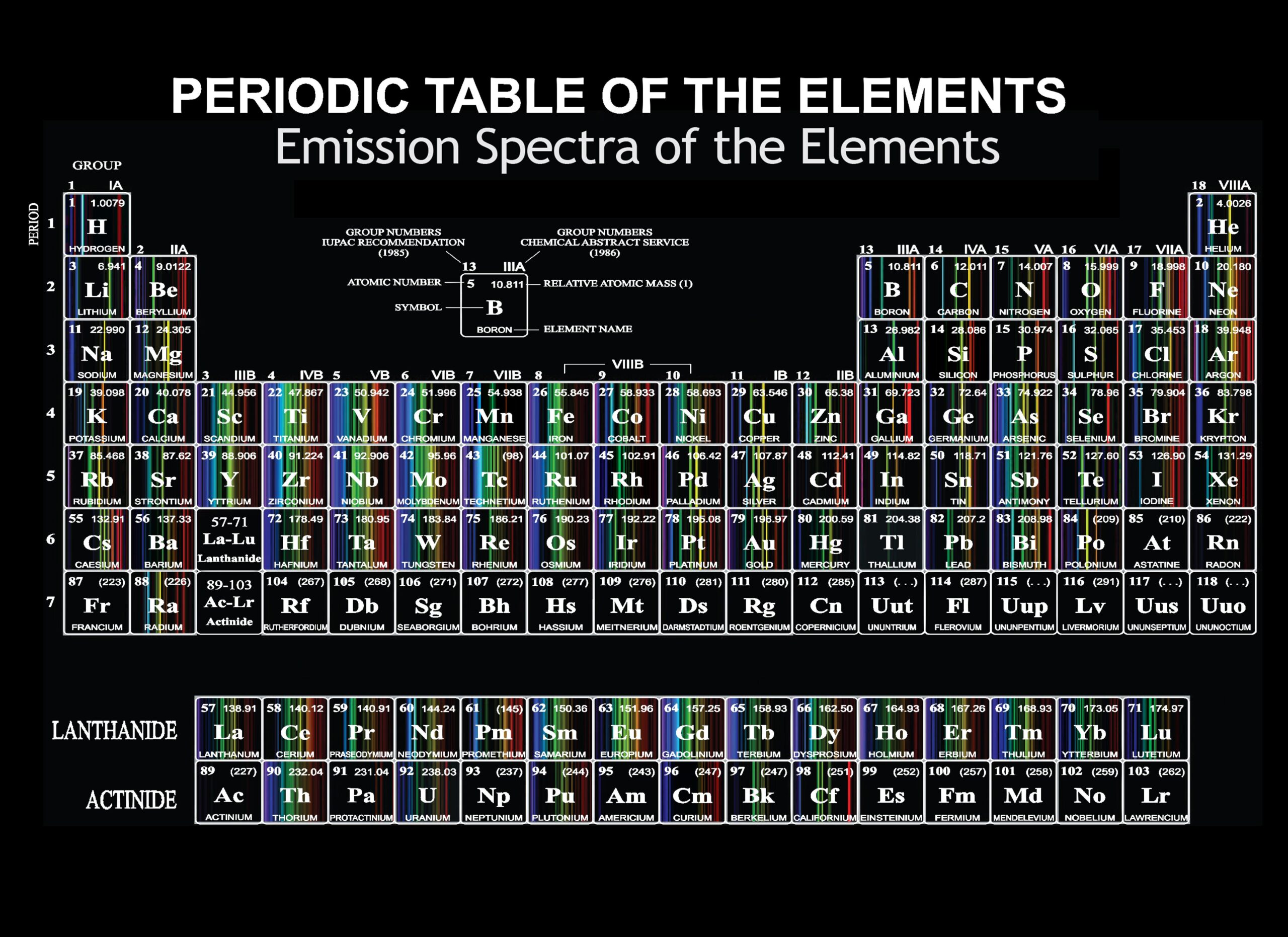
Emission Spectrum Periodic Table provides information about the wavelengths of light emitted by different elements. The Emission Spectrum Periodic Table is a valuable tool that displays the characteristic wavelengths of light emitted by various elements.
By examining the spectrum, scientists can identify elements based on their unique patterns of light emission. This information is essential for various fields, including astronomy, chemistry, and physics. The emission spectrum is determined by the energy levels within an atom, and as electrons transition between these levels, photons of distinct wavelengths are released.
By studying these emissions, scientists can gain a deeper understanding of atomic structure and the behavior of elements. We will explore the concept of emission spectra in relation to the Periodic Table, highlighting its significance in scientific research and beyond.
Credit: uwaterloo.ca
What Is An Emission Spectrum?
An emission spectrum refers to the specific wavelengths of light emitted by an element when it is heated or excited. These wavelengths can be observed and recorded on the periodic table, providing valuable information about the element’s properties.
Definition
An emission spectrum is a unique pattern of light emitted by an object or substance when it is energized. It is characterized by a series of spectral lines corresponding to specific wavelengths of light. These spectral lines are created when electrons within the object or substance move from higher to lower energy levels, releasing photons in the process. Each element or compound has its own distinct emission spectrum, which can be observed and analyzed using spectroscopy.
Importance
The emission spectrum plays a crucial role in scientific research and various fields of study. Here are some significant reasons why the emission spectrum is important:
- Identification of Elements: By analyzing the emission spectrum of a substance, scientists can determine the elements present in that substance. Different elements exhibit unique spectral lines, allowing for precise identification even in trace amounts. This is particularly useful in fields such as chemistry, astronomy, and forensic science.
- Study of Energy Levels: The emission spectrum provides valuable insights into the energy levels of atoms and molecules. By observing the specific wavelengths of light emitted, scientists can deduce the energy transitions occurring within the substance. This helps in understanding the electronic structures and behavior of particles.
- Chemical Analysis: Spectroscopy techniques based on emission spectra are widely used in chemical analysis. They enable scientists to measure concentrations, detect impurities, and monitor reactions in real-time. This aids in quality control, environmental monitoring, and various industrial processes.
- Astrophysics and Cosmology: The emission spectra of celestial objects, such as stars and galaxies, hold vital clues about their composition, temperature, and motion. By analyzing these spectra, astronomers can decipher the elemental abundances, temperature gradients, and even the presence of dark matter in the universe.
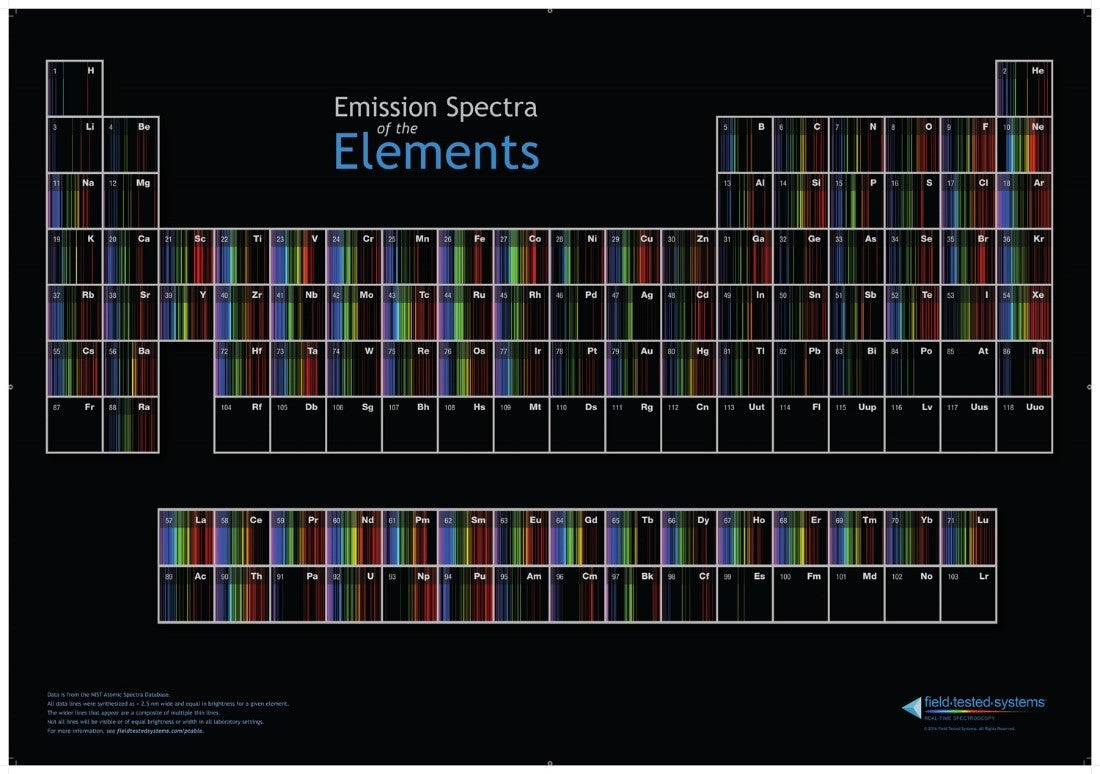
Credit: umop.net
Understanding The Periodic Table
The emission spectrum periodic table provides a comprehensive understanding of the elements’ light emission patterns, offering valuable insights into their atomic composition and behavior. Discover the intricate relationship between light and matter through this intuitive visual representation.
Understanding the Periodic Table The Periodic Table is a fundamental tool for chemists and students studying chemistry. It provides a visual representation of the elements, organizing them in a way that allows us to make sense of their properties and behaviors. In this section, we will explore the basics of the Periodic Table and how it is organized, shedding light on its importance in understanding the world of chemistry. H3: Basic Overview The Periodic Table is like a cheat sheet for chemists, containing all the information they need about the various elements. It displays the elements in a systematic manner, arranging them according to their atomic number, atomic mass, and electron configuration. With just a glance at the table, chemists can quickly gather essential information about an element, such as its symbol, atomic number, and atomic mass. H3: How it is Organized The organization of the Periodic Table is not arbitrary but follows a specific pattern. It is divided into sections, including periods and groups, that help us understand the characteristics and trends of elements. 1. Periods: The rows in the Periodic Table are called periods. There are seven periods in total, each representing a different energy level or shell of electrons. As you move from left to right across the periods, the number of electrons in the outermost shell increases by one. This arrangement allows chemists to observe trends in the properties of elements as they progress through the periods. 2. Groups: The columns in the Periodic Table are called groups. There are 18 groups in total, and each group contains elements that share similar properties. Elements in the same group tend to have the same number of electrons in their outermost shell, resulting in similar chemical behaviors. For example, elements in Group 1 (the alkali metals) all have one electron in their outermost shell, making them highly reactive and prone to forming positive ions. 3. Block Structure: The elements in the Periodic Table are further categorized into blocks based on their electron configurations. These blocks include the s-block, p-block, d-block, and f-block. The s-block consists of Group 1 and 2 elements, the p-block contains the groups on the right side, the d-block features the transition metals, and the f-block contains the rare earth elements. By organizing the elements in this way, the Periodic Table allows us to uncover the relationships between different elements and predict their behaviors. As we explore the table further, we will discover the significance of electron configurations and uncover the patterns and trends that underlie the behavior of the elements. In conclusion, understanding the Periodic Table is essential for anyone studying chemistry. By examining its basic overview and organization, we can begin to decipher the fascinating world of elements and their properties. Whether you are a student preparing for an exam or a chemist working in a laboratory, the Periodic Table will always be a valuable tool at your fingertips.Relationship Between Emission Spectra And Elements
Emission spectra offer valuable insights into the unique characteristics of elements, illustrated by the emission spectrum periodic table. By analyzing the emitted light from different elements, scientists can identify their elemental composition and understand their properties.
Emission spectra and elements have a deep-rooted relationship that scientists have been studying for centuries. By analyzing the light emitted by different elements, researchers are able to identify the distinct patterns and signatures associated with each element.
emission Spectra Patterns
When an element is heated or energized, it emits light of specific wavelengths, creating a unique emission spectrum. These emission spectra patterns are like a fingerprint for each element, making them invaluable in the field of spectroscopy.
Using emission spectra, scientists can identify elements with remarkable precision. By comparing the patterns in an unknown sample to the recorded spectra of known elements, researchers can determine the presence and concentration of specific elements in various substances. This methodology is especially useful in fields such as chemistry, astronomy, and environmental science.
To aid in the identification process, scientists have organized the emission spectra patterns of elements into a comprehensive tool known as the Emission Spectrum Periodic Table. This specialized version of the periodic table provides a visual representation of the unique emission spectra patterns for each element, simplifying the identification process.
While emission spectra can vary in complexity, there are some general patterns that can be observed:
- Continuous Spectrum:
A continuous spectrum is obtained when all wavelengths of light are emitted. This type of emission is typically observed in heated solids or dense gases. - Line Spectrum:
A line spectrum occurs when only specific wavelengths of light are emitted, resulting in distinct lines in the spectrum. This is the most common type of emission spectrum and is associated with individual elements. - Band Spectrum:
A band spectrum is characterized by a broad range of wavelengths being emitted, giving a continuous band of colors. This type of spectrum is often observed in complex molecules.
Understanding these emission spectra patterns is crucial for accurately identifying elements within a sample. By analyzing the unique patterns and comparing them to the emission spectrum periodic table, scientists can unlock valuable insights into the composition and properties of various substances.

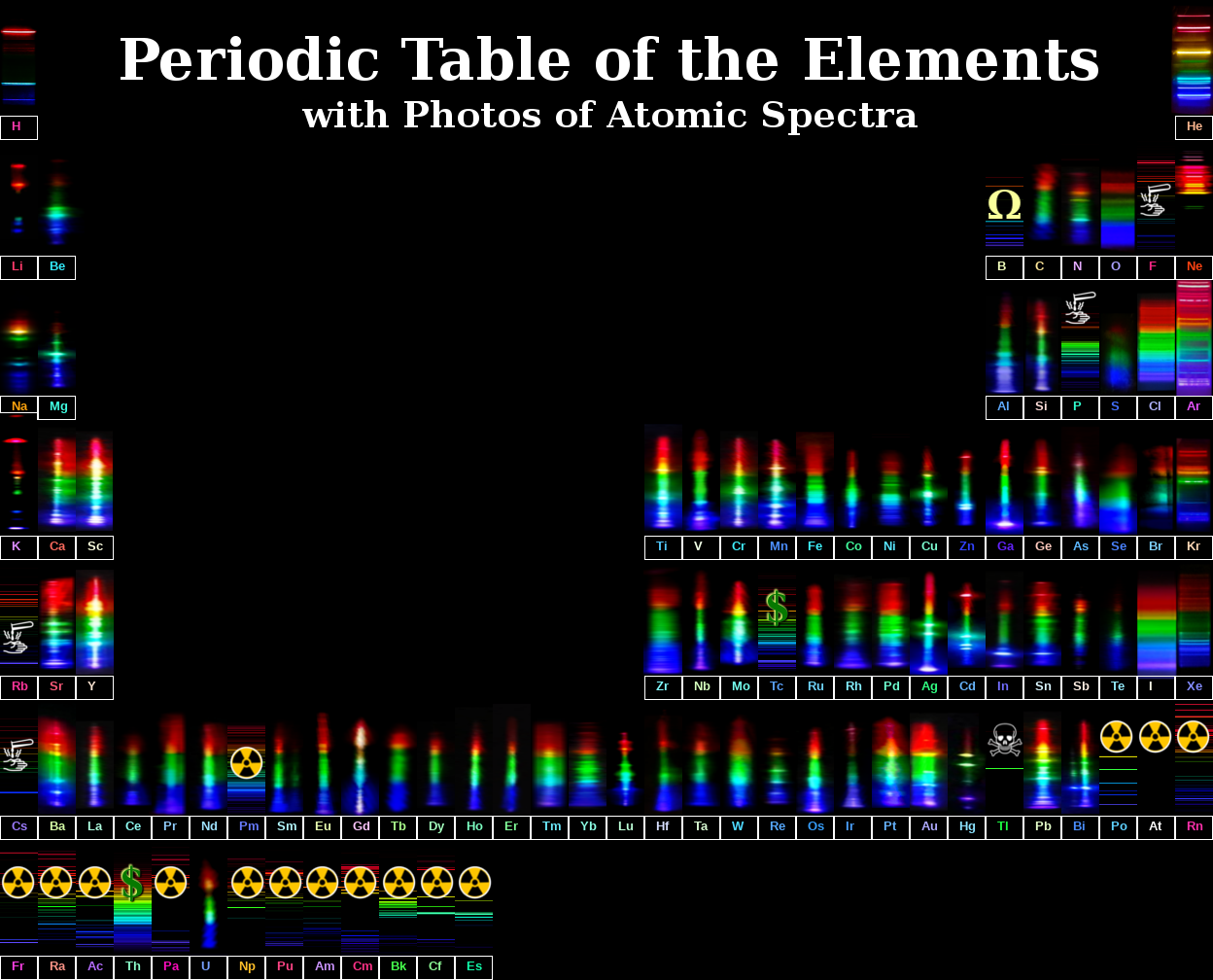
Credit: uwaterloo.ca
Applications Of Emission Spectroscopy
In the field of science, one of the most fascinating and useful techniques is emission spectroscopy, which allows scientists to analyze the properties of different elements based on their light emission. By studying the emission spectrum of an element, scientists can gain valuable insights into its composition, temperature, and even its distance from us.
Elemental Analysis
One of the key applications of emission spectroscopy is in elemental analysis. Scientists can use this technique to determine the presence and concentration of various elements in a sample. By measuring the intensity and wavelength of the emitted light, they can identify the elements present in the sample and even quantify their amounts. This information is crucial in fields such as environmental science, materials science, and forensic analysis.
Astronomy
In the vast expanse of the universe, emission spectroscopy plays a vital role in our understanding of distant celestial bodies. Astronomers use this technique to study the light emitted by stars, galaxies, and other cosmic objects. By analyzing the emission spectra, astronomers can glean valuable information about the chemical composition, temperature, and velocity of these objects. Furthermore, emission spectroscopy has been instrumental in the discovery of new elements in space, expanding our knowledge of the universe.
Future Developments And Research
As the understanding of emission spectra continues to evolve, ongoing research and developments are driving advancements in the analysis and applications of emission spectrum data. These advancements hold great potential for numerous fields, from environmental monitoring to materials science.
Advancements In Emission Spectrum Analysis
In the future, we can expect advancements in the analysis of emission spectra, with a focus on enhancing the accuracy and sensitivity of measurements. Researchers are constantly exploring new techniques and technologies that can improve the detection and quantification of specific elements in complex samples.
One area of research focuses on the development of novel spectroscopic instruments capable of capturing emission spectra with higher resolution and precision. These instruments will enable scientists to gain deeper insights into the composition and properties of substances, helping them understand the behavior of elements in various environments.
Additionally, the advancements in emission spectrum analysis will likely incorporate machine learning and artificial intelligence algorithms, allowing for faster and more efficient data processing. These algorithms can greatly enhance the identification of emission lines and improve the accuracy of element identification, even in the presence of noise or overlapping spectra.
Potential Applications
The future of emission spectrum analysis holds immense potential for a wide range of applications across different industries. From environmental monitoring to industrial processes, the ability to accurately analyze emission spectra can provide valuable insights and contribute to various scientific and technological advancements.
One potential application lies in environmental monitoring and pollution control. By analyzing emission spectra from various sources such as exhaust gases or industrial emissions, scientists can detect and quantify harmful pollutants. This knowledge can then be used to develop effective mitigation strategies and improve the quality of our environment.
Moreover, emission spectrum analysis can play a vital role in material science and engineering. By studying the emission spectra of different materials, researchers can understand their atomic and molecular structure. This understanding can lead to the development of novel materials with specific properties and functionalities, benefiting industries such as electronics, energy storage, and catalysis.
In conclusion, the future developments and research in emission spectrum analysis are poised to revolutionize our understanding of elements and their behavior. With advancements in analysis techniques and potential applications in various fields, we can look forward to new discoveries and innovations driven by the insights gained from emission spectra.
Frequently Asked Questions Of Emission Spectrum Periodic Table
What Is An Emission Spectrum?
An emission spectrum is a unique pattern of light emitted by an element or a substance when it is excited or heated. It consists of distinct colored lines or bands that correspond to specific wavelengths of light.
How Is The Emission Spectrum Related To The Periodic Table?
The emission spectrum of an element is directly related to its atomic structure, which is represented in the periodic table. Each element has a unique set of energy levels for its electrons, and when these electrons transition between levels, they emit light at specific wavelengths, leading to the characteristic emission spectrum.
Can The Emission Spectrum Be Used To Identify Elements?
Yes, the emission spectrum is widely used in analytical chemistry to identify elements. Each element has a unique emission spectrum, acting as its “fingerprint. ” Comparing the observed emission spectrum of an unknown sample with known spectra can help determine the elements present in that sample.
How Does The Emission Spectrum Help In Understanding Atomic Structure?
The emission spectrum provides valuable information about the energy levels of electrons in an atom. By analyzing the wavelengths and intensities of the emitted light, scientists can deduce the specific energy transitions that occur within the atom, thereby gaining insights into its electronic structure and the arrangement of its orbitals.
Conclusion
Understanding the emission spectrum periodic table is crucial for scientists and researchers in various fields. By studying the unique wavelengths of light emitted by different elements, we can gain valuable insights into their atomic structure and properties. This knowledge serves as a foundation for advancements in areas such as chemistry, physics, and astronomy.
Embracing the intricacies of the emission spectrum periodic table opens up a world of possibilities for exploring the fundamental building blocks of our universe.